
Manufacturing Facility
We are world class producer of Generic Pharmaceuticals products.


Manufacturing Facility
We produce quality products at its state of the art formulation plant. A highly qualified and trained team of scientist, pharmacists and Chemist work in our Research and Development and operations department, developing new Products in an environment that celebrates continuous improvement in quality. Our manufacturing site has the latest analytic instruments. We monitor quality assurance through quality systems that ensure the consistent quality of the products.
We always encourage all the staff to produce high quality products by using technology in accordance with cGMP guidelines and WHO standards. The production process is supervised by highly qualified professional with vast experience in the field. Biogenetic Drugs plant is constantly inspected and approved for the production of Pharmaceuticals accordance with cGMP.
The Biogenetic Drugs areas are special places where only authorized personnel with proper gear can enter. These areas have advance Heating, Ventilation & Air Condition (HVAC) systems with separate zoning. These are usually dust proof, temperature, and humidity controlled. The manufacturing personnel and visitors have to wear lab coats, caps and face mask to cover their hair, beards, and foot covers for shoes. These stringent hygiene requirements are to ensure reliable and consistent product quality free from impurities.
Facility Description
Biogenetic Drugs is a medium sized Pharmaceuticals industry with a manufacturing site at Solan, Himachal Pradesh. The numbers of employees approximately are 150. Total covered area is 16314 square feet.
a. Biogenetic Drugs places basic emphasis on the concept of quality and on strict compliance with the rules of current Good Manufacturing Practices in all steps of manufacturing, testing, storage, shipping and marketing.
b. The manufacturing facility of Biogenetic Drugs is designed to manufacture Tablets, Capsules and Dry powder Suspensions products.
c. Plant operations are administered through the Quality and Production Division:
i. Quality Division
1. Quality Assurance
2. Quality Control
ii. Production Division
1. Production
2. Packaging
3. Warehousing
iii. There is a main reception and a canteen.d. Production line capacity of Biogenetic Drugs per single shift / 24 hour / 5 days working is as follows:
i. Tablets: 666 million tablets annually.
ii. Capsules: 76 million capsules annually.
iii. Dry Powdered Suspension: 76 million bottles annually
Compressed Air
The plant has one oil free air compressor, with a capacity of 1538 l/min. the oil free compressed air is supplied to a tank of 350 litres capacity.
Electric Power
Biogenetic Drugs total peak power consumption is about 85 KW and is obtained from LEPCO (Lahore Electric Power Company). Biogenetic Drugs also has one standby generator, 100 KVA, which is to be used during main power failure.
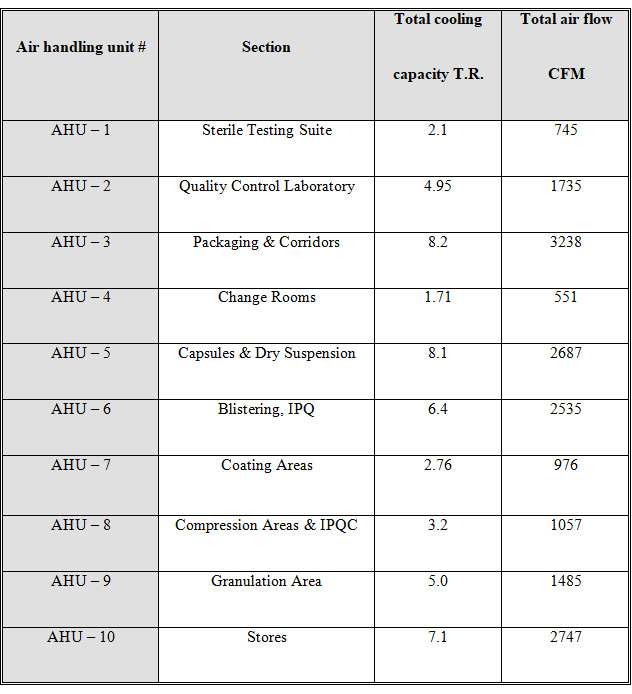
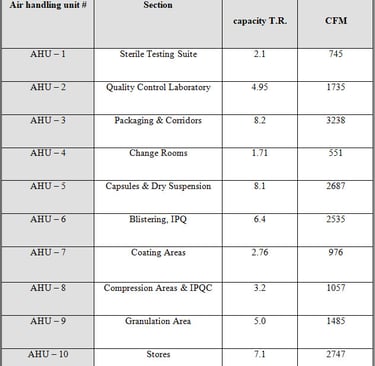
HVAC System
Building of Biogenetic Drugs has one HVAC system with ten separate air handling units (AHU). All the air handling units are installed to control the contamination. Lay outs of AHUs are attached. Detail of HVAC system is as follows:
BIOGENETIC DRUGS PRIVATE LIMITED
Factory Address: Plot No. 79 A & B, Village Thana, Baddi, Himachal Pradesh 173205
Phone: +91 95928 23673, 82641 98296 | E-mail: mailme@biogeneticdrugs.com | Web: www.biogeneticdrugs.com
CIN: U24232RJ2004PTC018909 | GSTIN: 02AACCB3897K1ZJ


Registered Office: Near Micro Turner, Jharmajri, Baddi, Solan, Himachal Pradesh, 173205
